Teleflex Rusch 850401 Urine Bag 2l 30 Pieces

- Brand: TELEFLEX MEDICAL Srl
- Product Code: 902020116
- EAN:
- Availability: In Stock
- Purchase 3 items for 17.34€ each
- Purchase 4 items for 16.98€ each
- Purchase 5 items for 16.63€ each
Teleflex
Rusch
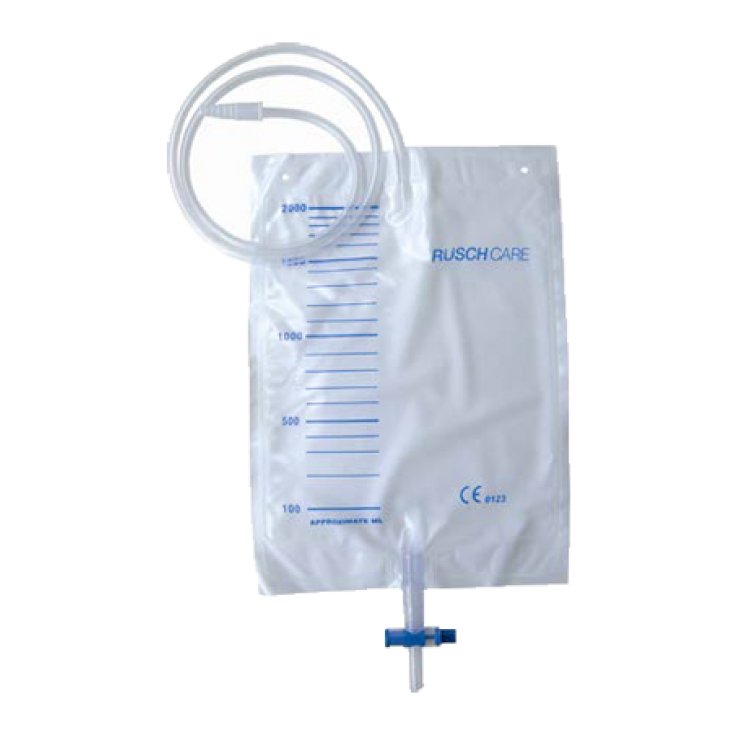
Comfort Urine Bag
BY BED
CE Class I medical device. ISO code: 092707003. Repertory no.: 91702 / R.
Urine collection bag with connecting tube, non-return and drain valve T-Tap, pre-printed volume indication. With a support for the bed.
Components
Construction material: PVC.
Latex: no.
DEHP: yes.
Medicines: no.
Substances: no.
Organic fabrics: no.
The bag is a single piece made of PVC with special and patented physiologically harmless emollients and stabilizers. The bed support is made of PVC. The materials are controlled according to USPXXIII, class VI and ISO 10993, EN30993 standards.
Sterilization
Product validity: 5 years from the date of manufacture.
Non sterile and non resterilizable single use product.
Physical - chemical incompatibility of materials
Chemical-physical incompatibility of materials towards substances with which they may potentially come into contact:
- Fats and oils, such as Vaseline and paraffin oil;
- Organic solvents such as benzene and ether;
- Oxidizing materials such as hydrogen peroxide, sulfur hypochlorite;
- Disinfectants containing phenol or similar.
Packaging methods
The packaging of the product has been designed to allow a good conservation of the same and easy storage by overlapping. Each package clearly and legibly reports, in Italian, the qualitative and quantitative description of the content, the name of the manufacturer and any other useful information for immediate recognition of the product itself. The individual packages are easy to open, such as not to allow the material to adhere to the package, facilitating the removal of the product.
Material used for primary packaging
The product is packaged in a package made from a low density polyethylene layer and a reinforced lacquered paper film, also guaranteeing excellent resistance to impact or traction.
Method of conservation
Store at room temperature, away from direct exposure to light. Prolonged exposure to fluorescent light, sunlight or heat will damage the device.
Disposal methods
Check the indications dictated by the Italian regulations regarding the disposal of medical devices.
Quality checks
The devices are designed and manufactured in such a way that their use does not compromise the clinical status and safety of patients, nor the safety and health of users and possibly third parties, when used under the conditions and for the intended purposes. The quality control, carried out according to international standards, provides for a series of checks at each stage of production.Each production batch undergoes constant technical-chemical-biological control from the moment of use of the raw material to sterilization. The certified company operates according to a Quality System in accordance with GMP, EN ISO14001, ISO 13485, EN 550 and according to MDD 93/42.
Format
Tube length: 90 cm; tube diameter: 5x7 mm; capacity: 2000 cc. 30 pieces, envelope.
Code 850401