SARS-CoV-2 Leading ALL TEST 20 Test Antigen Rapid Test
- Brand: LEADING MED Srl
- Product Code: 983376601
- EAN:
- Availability: Out Of Stock
- Purchase 3 items for 73.63€ each
- Purchase 4 items for 72.12€ each
- Purchase 5 items for 70.62€ each
Rapid Antigen Test SARS-CoV-2 Leading ALL TEST - Medical Device
The COVID-19 Antigen Rapid Test Cassette (Oral Fluid) is a single-use test kit intended to detect the novel coronavirus SARS-CoV-2 that causes COVID-19 in human oral fluid. The test is designed for home use with self-sampling of oral fluid. The test is intended for use in symptomatic individuals who meet the case definition for COVID-19 as well as in asymptomatic individuals, limited to contacts of confirmed or probable cases of COVID-19 and healthcare professionals at risk.
The COVID-19 Antigen Rapid Test Cassette (Oral Fluid) provides a preliminary result only, final confirmation must be based on clinical diagnostic results.
How does AllTest Salivary Rapid Test for Covid-19 Antigen work?
The new coronavirus belongs to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally sensitive. At present, patients infected with the new coronavirus are the major source of infection; even asymptomatic infected people can be a source of infection. Based on current epidemiological investigations, the incubation period ranges from 1 to 14 days, in most cases from 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are present in a limited number of cases.
(PRINCIPLE)
The COVID-19 Rapid Antigen Test (Oral Fluid) is a qualitative membrane-based immunoassay for the determination of SARS-CoV-2 antigens in human oral fluid samples.
(REAGENTS)
The test device contains SARS-CoV-2 antibodies.
How to use
Before taking the test
Do not put anything in your mouth, including civo, beverages, chewing gum, or tobacco products for at least 10 minutes prior to testing.
Wash your hands with soap and water for at least 20 seconds before testing. If soap and water are not available, use hand sanitizer with at least 60% alcohol.
Step 1: Collect the sample
Remove the funnel and the plastic tube from the packaging, insert the funnel on the tuber.
Cough deeply 3-5 times.
Note: Wear a face mask or cover your mouth and nose with a cloth while coughing and keep distance from bystanders.
Gently spit the oral fluid into the funnel.
The oral fluid (without bubbles) should reach the height indicated by the reference line .
NOTE: If insufficient fluid is collected, repeat the sample collection steps outlined above.
Insert the used funnel into the bio-safety bag.
Step 2: Sample Preparation
Pull to open the buffer solution and pour all the solution into the tube with the oral fluid. Insert the cap on the tube. Gently squeeze the tube 10-15 times to mix well.
Step 3: Run the test
Remove the test device from the sealed pouch and use it within one hour. The best results are obtained if the test is performed immediately after opening the foil pouch.
Place the test cassette on a flat surface.
Invert the tube and pour 2 drops of solution into the well (S) of the test device and then start the timer.
Do not move the test cassette while the test is running.
Step 4: Read the results after 15 minutes
Do not interpret the results after 20 minutes.
After completing the test, place all test kit components in the bio-safety bag and dispose of it according to local regulations. Do not reuse any of the components of the used kits.
Wash your hands thoroughly after disposing of the test.
Reading the results
Inform your doctor of the results and carefully follow local instructions / guidelines for COVID cases.
POSITIVE: * Two colored lines appear.
One colored line should appear in the control region (C) and another colored line should appear in the test region (T).
* NOTE: The intensity of the color in the test line region (T) varies depending on the number of SARS-CoV-2 antigens present in the sample. Therefore the presence of any shade of color in the test region (T) should be interpreted as a POSITIVE result. A positive result means that it is very likely that the person is infected with COVID-19, positive samples must be confirmed. Immediately self-isolate as prescribed by local regulations and contact your treating physician or health care district as directed by local authorities. The test will be verified by a confirmatory molecular test (PCR) and instructions on the following steps will be provided.
NEGATIVE: only one colored line appears in the control region (C).
No colored lines appear in the test line region (T). It is unlikely that you have COVID-19. However, it is possible that this test will give an incorrect result (a false negative) in some people with COVID-19. This means that you may have contracted COVID-19 even if the test gave a negative result. In case of symptoms such as headache, migraine, fever, loss of taste and smell, please contact the nearest health care point according to local authority rules. Also, you can repeat the test with a new kit. If suspected, repeat the test after 1-2 days, as the coronavirus cannot be determined at all stages of the infection. Even with a negative result, the rules of spacing and hygiene must continue to be observed. Migration / travel, participation in events and so on should follow local COVID guidelines / requirements.
INVALID: The control line does not appear.
Insufficient sample volume or incorrect procedure are the most likely causes of missing control line. Review the procedure and repeat the test with a new kit or contact your doctor or a COVID-19 diagnosis center.
Limitations
- Failure to follow some steps of the test procedure may result in incorrect results.
- The COVID-19 Antigen Rapid Test Cassette (Oral Fluid) is intended for in vitro self-diagnosis only.
- The results obtained with the test should be considered in addition to other clinical results of other tests and laboratory evaluations.
- If the test result is negative or non-reactive but clinical symptoms persist, it is because the virus may not be detectable in the early stage of infection. It is recommended to repeat the test with a new test 1-2 days later or to go to the hospital to rule out infection.
- Positive COVID-19 results may be due to infection with non-SARS-CoV-2 virus strains or other interfering factors.
Warnings
- Read the package insert carefully before performing the test.
- The test is intended for in vitro self-diagnosis only.
- Test is single use, do not reuse. Do not use after the expiration date.
- Do not eat, drink or smoke in the area where specimens or kits are handled.
- Do not drink the buffer solution from the kit. Handle the buffer solution with care and avoid contact with skin and eyes, in case of contact rinse immediately with plenty of running water.
- Do not use the test if the package is damaged.
- Wash hands thoroughly before and after handling.
- If the preliminary result is positive, inform your doctor of the result and carefully follow the local instructions / regulations for COVID cases.
- Tests performed on children and adolescents must be carried out in the presence of adults.
- Used test must be disposed of according to local regulations.
storage
Store the test at temperatures between 35.6-86 ° F (2-30 ° C).
Do not open the package until you are ready to test.
DO NOT FREEZE
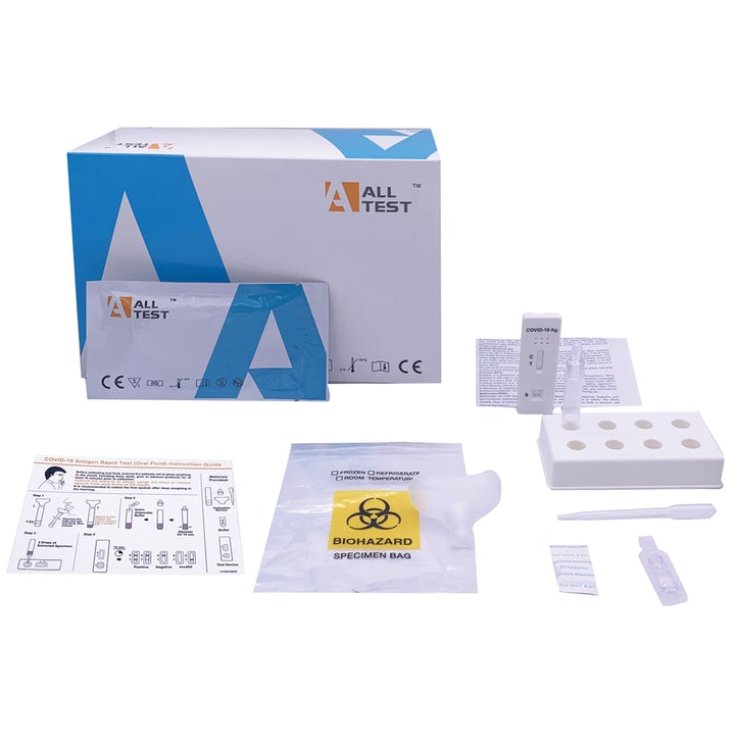
Format
- Test device;
- Sample collection device (funnel, tube and tube tip);
- Buffer solution;
- Package leaflet;
- Bio-security bag.