Flair 2 Uro Plate 45 Rit13-45 5 Pieces

- Brand: TELEFLEX MEDICAL Srl
- Product Code: 920375488
- EAN:
- Availability: In 10 - 14 Days
- Purchase 3 items for 20.34€ each
- Purchase 4 items for 19.92€ each
- Purchase 5 items for 19.50€ each
Flair 2 Uro
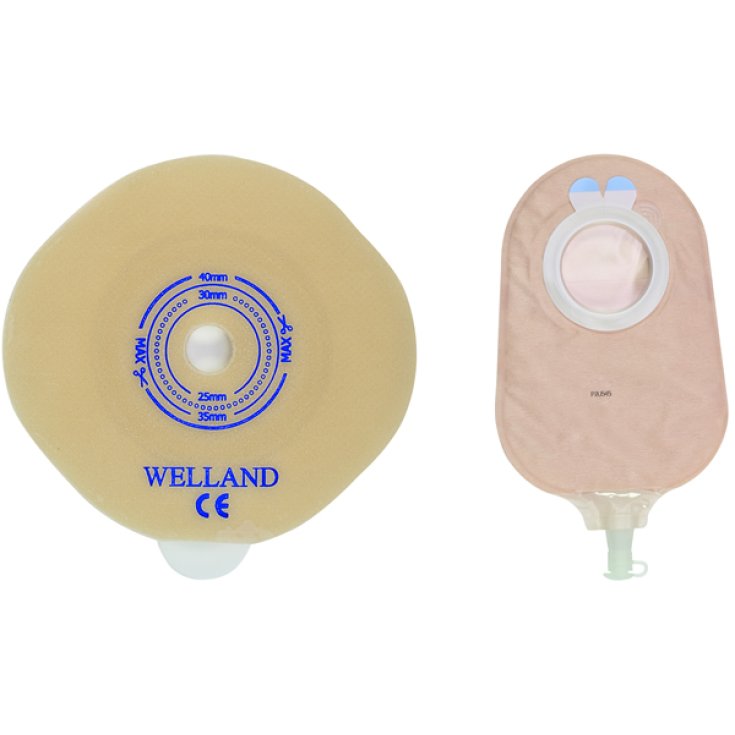
TWO-PIECE SYSTEM UROSTOMY FLAT PLATE
Class I medical device.
Hydrocolloid plate to be adhered to the peristomal skin to be connected to the relative bag (supplied with another code) for the collection of excreta for urostomized patients. Plate with flange for urostomy for two-piece system, equipped with a self-supporting protective barrier, made of hydrocolloid in order to guarantee a good seal and excellent tolerability on the part of the patient's skin.The plate is equipped with a PVC flange for connection to the relative bag (supplied with another code) and safety adhesive system to avoid any accidental detachment.
Nutritional characteristics
| Product code | Description | Diameter mm |
| R011PP45URO-RT | Flat Plate Flair2 Urostomy, mm45 | Cuttable 13 - 45 |
| R011PP45URO-25 | Flat Plate Flair2 Urostomy, mm45 | Pre-cut 25 |
| R011PP45URO-29 | Flat Plate Flair2 Urostomy, mm45 | Pre-cut 29 |
| R011PP45URO-32 | Flat Plate Flair2 Urostomy, mm45 | Pre-cut 32 |
| R011PP45URO-35 | Flat Plate Flair2 Urostomy, mm45 | Pre-cut 35 |
| R011PP45URO-38 | Flat Plate Flair2 Urostomy, mm45 | Pre-cut 38 |
| R011PP55URO-RT | Flat Plate Flair2 Urostomy, mm55 | Cuttable 13 - 55 |
Construction material
Hydrocolloid; PVC; non-woven fabric.
Sterilization specifications
Product validity: 5 years from the date of manufacture.
Non sterile and non resterilizable single use product.
Physical - chemical incompatibility of materials
Chemical-physical incompatibility of materials towards substances with which they can potentially come into contact:
Fats and oils, such as Vaseline and paraffin oil;
Organic solvents such as benzene and ether;
Oxidizing materials such as hydrogen peroxide, sulfur and hypolchlorite;
Disinfectants containing phenol or similar.
Packaging methods
The packaging of the product has been designed to allow a good conservation of the same and easy storage by overlapping. Each package clearly and legibly reports, in Italian, the qualitative and quantitative description of the content, the name of the manufacturer and any other useful information for immediate recognition of the product itself. The individual packages are easy to open, such as not to allow the material to adhere to the package, facilitating the removal of the product.
Material used for primary packaging
The product is packaged in a PVC blister in order to guarantee its correct conservation during transport and storage, with a suitable method of use.
storage
Store at room temperature, away from direct exposure to light. Prolonged exposure to fluorescent light, sunlight or heat will damage the device.
Disposal method
Check the indications dictated by the Italian regulations regarding the disposal of medical devices.
Format
5 pieces.