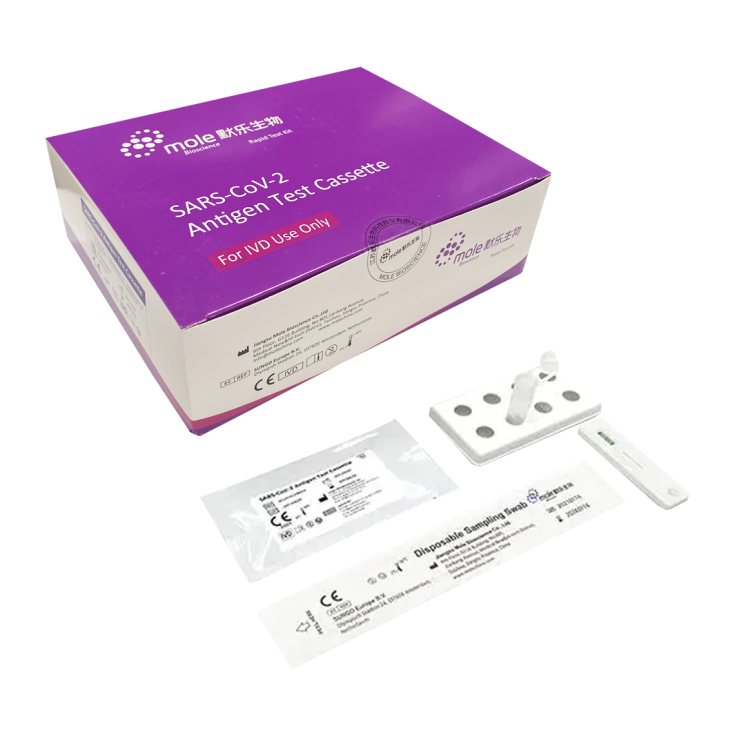
Mole Bioscence Kit 20 Antigen Rapid Tests for SARS-CoV-2

- Brand: ALPHA PHARMA SERVICE Srl
- Product Code: 963581285
- EAN:
- Availability: Out Of Stock
- Purchase 3 items for 60.09€ each
- Purchase 4 items for 58.87€ each
- Purchase 5 items for 57.64€ each
Mole Bioscence Kit 20 Antigen Rapid Tests for SARS-CoV-2
20 SARS-CoV-2 Rapid Antigen Detection Tests Kit Rapid chromatographic immunoassay kit for the qualitative detection of SARS-CoV-2 antigens from nasal swab specimens to facilitate rapid diagnosis of SARS-CoV-2 viral infections. For professional in vitro diagnostic use only. Jiangsu Mole Bioscience brandMANUFACTURER'S DECLARATION The manufacturer Mole Bioscence declares that the rapid antigen test is not altered in case of infection with SARS-CoV-2 latest variant “Omicron” and other variants. The Manufacturer can confirm that the test is suitable for detecting these variants of SARS-CoV-2
Characteristics
Antigenic Rapid Pharyngeal Nose Test SARS-CoV-2 detection
The SARS-CoV-2 Rapid Pharyngeal Nose Antigen Test is a rapid immunochromatographic test for the qualitative detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigens in human nasal swab specimens. The test is intended to aid in the rapid differential diagnosis of SARS-CoV-2 viral infection in vitro.
Principle of the Antigenic Rapid Nasal Test
The SARS-CoV-2 Antigen Test Cassette is an immunoassay based on the immunochromatographic principle for the determination of the SARS-CoV-2 antigen extracted from the nasal swab sample. The test strip consists of the following parts: Sample Buffer, Reagent Buffer, Reaction Membrane, and Absorbent Buffer. The reactive buffer contains colloidal gold conjugated with monoclonal antibodies against the SARS-CoV-2 core capsid protein.
What materials are provided with the Rapid Pharyngeal Nose Test?
- Cassette test: 20
- Disposable swab: 20
- Extraction buffer: 20
- Test tube with dropper cap: 20
- Instructions for use: 1
- Work station: 1
STORAGE AND DURATION
- Store at 4-30 ° C in the sealed pouch until the expiration date printed on the package. Do not freeze.
- The delt est cassette must be used within 1 hour of opening the sealed pouch.
- The contents of the kit are stable until the expiration date printed on the package.
- Keep away from sunlight, humidity and heat sources.
Collection and preparation of the nasal sample
- Tilt the patient's head back 70 degrees.
- While gently rotating the swab, insert the pad, less than one inch (approximately 2 cm) in size, into the nostril parallel to the palate (not upward) until resistance to the turbinates occurs.
- Rotate the swab at least 5 times against the nasal wall and then remove it by rotating it slowly.
- Repeat the previous steps in the other nostril using the same swab. The swab collected with the sample should be tested as soon as possible. If immediate testing is not possible, the swab should be placed in a sterile plastic tube labeled with patient information and airtight at room temperature (15-30 ° C) until 1 hour prior to testing.
Test procedure from nasal and nasopharyngeal samples
- Allow the Test Cassette, specimen, buffer, and / or controls to reach room temperature of 15-30 (59-86 ℉) prior to testing.
- Place the tube on the workstation. Add all extraction buffer solutions to the extraction tube.
- After sample collection, insert the swab into the extraction tube that contains the swab and swirl the swab for approximately 10 seconds to dissolve sufficient sample within the swab.
- Remove the swab by pressing the end of the swab to unscrew the remaining sample.
- Dispose of the swab according to the biohazard waste disposal protocol.
- Remove the test cassette from the laminated pouch by tearing the notch and place it on a clean, horizontal surface.
- Cover the extraction tube with the cap and add 3 drops of the sample vertically into the sample well of the cassette.
- Read result after 15 minutes The test result should not be read and interpreted after 20 minutes.
A procedural check is included in the test. A colored line appearing in the control line area (C) is considered an internal procedural control. It confirms sufficient sample volume, adequate membrane humidification and correct procedural technique. Quality Controls (Positive Control Buffer and Negative Control Buffer) are not included in the kit and can be purchased separately.
Interpretations of the results
- Positive: Two bands appear in the results window: test band (T) and control band (C) and the test is considered positive for SARS-CoV-2 antigen.
- Negative: If only the control band (C) appears in the result window, the test is considered negative for the SARS-CoV-2 antigen
- Invalid: If the control band (C) does not appear, even if the test band (T) appears in the test area, it is considered an invalid result, review the procedure and repeat the test with a new test cassette. If the problem persists, stop using the test kit immediately and contact your local distributor
Format
20 Quick Test Kit.