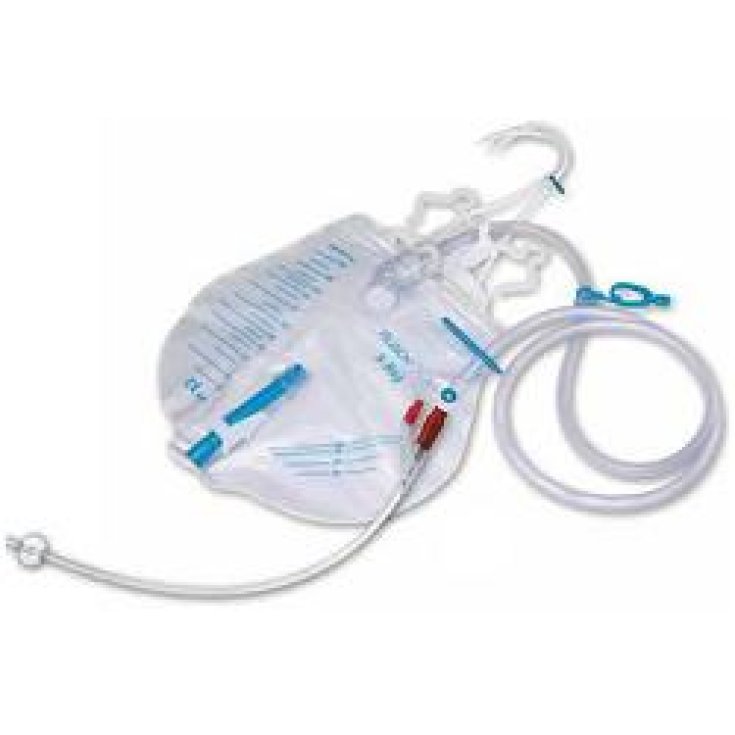
PRE-CONNECTED STAY BLADDER CATHETER KIT CH18 TeleFlex®

- Brand: TELEFLEX MEDICAL Srl
- Product Code: 923510174
- EAN:
- Availability: In 10 - 14 Days
- Purchase 3 items for 34.44€ each
- Purchase 4 items for 33.73€ each
- Purchase 5 items for 33.03€ each
PRE-CONNECTED STAY BLADDER CATHETER CH 18 - COMPLETE WITH POSITIONING KIT
PROFILCATH PRECONNECTED
CE class IIb medical device.
Indications for use
For bladder catheterization.
Residence time
> 30 days.
From a clinical point of view, the actual residence time in situ is closely related mainly to the patient's condition, such as urine concentration and pH, infections, the patient's tendency to stone formation and metabolic deficiency. Based on these parameters, the residence time can vary from patient to patient. The residence time may vary from patient to patient.
Characteristics
Bladder catheter in pure 100% transparent silicone with longitudinal grooves for drainage of urethral secretions and for the reduction of decubitus, with funnel for syringes with Luer and Luer - Lock fittings, syringe of water and 10% glycerin for balloon inflation included, in radiopaque tip, with radiopaque line preconnected to urine collection bag with a capacity of 2000 ml, with drip tray, non-return valve, antibacterial filter, urine collection site without the use of a needle, indication of the pre-printed volume, tap concealed drainage system, with clamp, double bed support, 120cm long tube.
Model 851086 and system positioning kit model CMK01076 / PSC.
Available in sizes Ch. 14-16-18-20.
Presence of latex, phthalates (DEHP), drugs, substances, biological tissues
| Description | Yup | No |
| Latex | X | |
| DEHP | X | |
| Medicines | X | |
| Substances (*) | X | |
| Organic fabrics | X |
(*) Contains syringe with 10ml of sterile water.
Contains syringe with 10 ml sterile water and glycerin.
Contains 12.5ml lubricant.
Contains 0.05% chlorhexidine gluconate antiseptic.
Construction material
SILKOMED silicone 100% grooved, (catheter), PVC (bag), TNT (cloth), NITRILE, (gloves).
Material description
SILKOMED is the registered trademark of the silicone elastomer, used by TELEFLEX MEDICAL in the medical sector. It is chemically produced using inorganic raw materials. Its properties make it physiologically harmless (suitable for implants), resistant to aging, oxygen, ozone, UV rays and temperatures up to 200 ° C. The material is free of substances derived from water and organic fluids. SILKOMED is tested according to USPXXIII, class VI and ISO 10993, EN 30993 standards. Its distinctive features are water repellency and high resistance to blood and organic tissues.
| Name | Description |
| TWO-LAYER TNT, WATERPROOF AND ABSORBENT TOWEL 50cm x 60cm | Double-coupled sheet made with hot melt laminate, film: embossed multilayer based on Polyolefine. Weight 54gr / m2 Composition of the non-woven fabric: substrate A: chemical bonded viscose. |
| WATERPROOF AND ABSORBENT TWO-LAYER FENESTRATED TOWEL IN TNT, 50 cm x 60cm WITH HOLE 6cm | Double-coupled sheet made with hot melt laminate, film: Polyolefine-based embossed antistatic multilayer. Weight 54gr / m2 Composition of the non-woven fabric: substrate A: chemical bonded viscose. |
| 5 STERILE GAUZE TABLETS IN 4-LAYER FOLDED TNT 10x10 cm | Nonwoven gauze compresses made of 100% viscose, measuring 10cm x 10cm folded in 4 layers, weight 40gr. |
| MONODOSE CHLOREXIDINE-BASED ANTISEPTIC. BAXIDIN SKIN SOLUTION 25ml AIC 032158040 | BAXIDIN ready to use, medicinal product for self-medication. Indications: disinfection and cleaning of damaged skin. AIC holder of the SANITAS company |
| SYRINGE WITH 10ml OF PURIFIED WATER | Disposable syringe containing FUIX sterile purified water |
| 2 PAIRS STERILE NITRILE GLOVES, SIZE L | Nitrile glove, manufactured in compliance with ASTM D5250 quality standards. Sensitive ensures excellent protection in all conditions of use. |
| STERILE LUBRICANT IN BELLOWS PACKAGING WITH LIDOCAINE | Single-dose bellows containing lubricating gel for catheterization operations, construction materials: 1.2% hydroxymeticellulose, water for injections, hydrochloric acid, 0.05 chlorhexidine hydrochloride, 2% lidocaine hydrochloride. |
Duration of sterilization
4 years from the date of manufacture.
Sterilization
TELEFLEX and CME products are sterilized with ethylene oxide as per EN 550: 1994, EN ISO 10993-7: 1995 standards. Inscriptions indicating the sterilization date, expiration date and lot number appear clearly on the product packaging. Sterility is not guaranteed if the package is not intact. Use immediately after opening the package. Disposable product that cannot be resterilized.
Physical - chemical incompatibility of materials
Physical and chemical incompatibility of materials towards substances with which they can potentially come into contact:
- Fats and oils, such as Vaseline and paraffin oil;
- Organic solvents such as benzene and ether;
- Oxidizing materials such as hydrogen peroxide, sulfur hypochlorite;
- Disinfectants containing phenol or similar.
Method of packaging
The packaging of the product has been designed to allow a good conservation of the same and easy storage by overlapping. Each package shows in a clear and legible way, in Italian, the qualitative and quantitative description of the content, the name of the manufacturer and any other information useful for the immediate recognition of the product itself. The individual packages are easy to open, such as not to allow the material to adhere to the package, facilitating the removal of the product.
Material used for primary packaging
The primary packaging consists of a transparent part made of PVC and a part of medical paper.
Quantity for secondary packaging
A secondary pack contains 1 piece, individually wrapped.
Method of conservation
Store at room temperature, away from direct exposure to light. Prolonged exposure to fluorescent light, sunlight, or heat will damage the device.
Method of disposal
Check the indications dictated by the Italian regulations regarding the disposal of medical devices.
Quality Controls
The devices are designed and manufactured in such a way that their use does not compromise the clinical status and safety of patients, nor the safety and health of users and possibly third parties, when used under the conditions and for the intended purposes. The QUALITY CONTROL, carried out according to International Regulations, provides for a series of checks at each stage of production. Each production batch undergoes constant technical-chemical-biological control from the moment of use of the raw material to sterilization. The certified company operates according to a Quality System in accordance with GMP, EN ISO 14001, ISO 13485, EN 550 and according to MDD 93/42.
Method of use
Please refer to the instructions for use of the product.
CE certified
MED 31015 / 50076-16-04.
Declaration of conformity
DC-M018 (item 851086)
CMK01076 / PSC3-CHxx (art. CMK01076 / PSC).
No. Repertoire
38264.
CND
U0199 (art.851086)
T0202 (item CMK01076 / PSC).
GMDN
34917 (art. 851086)
N / A (art. CMK01076 / PSC).
Package Contents
Kit composed as follows:
- 1 two-layer non-woven fabric 50 x 60 cm.
- 1 two-layer non-woven fabric 50 x 60 cm with hole and cut.
- 5 4-layer tablets 10 x 10 cm.
- 1 single-dose disinfectant 25 ml (antiseptic).
- 1 10 ml syringe with purified water.
- 2 pairs of wrapped size L nitrile gloves.
- Bellows lubricating gel with chlorhexidine.
Pre-connected drainage system composed as follows:
Preconnected system consisting of indwelling pure silicone grooved catheter with closed circuit bag.
Product Code: 852086-000xxx.
Ch: 14/16/18/20.