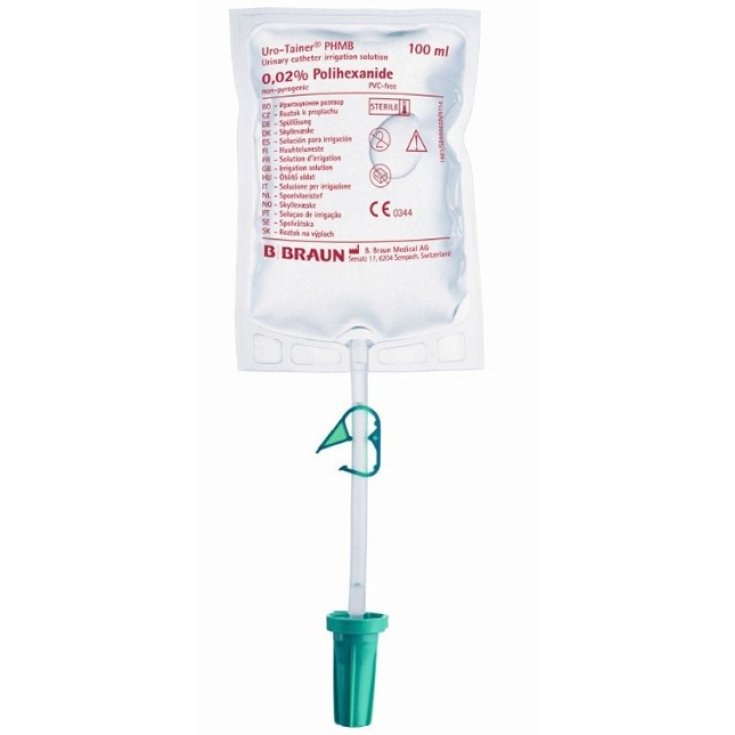
Uro-tainer Poliesanide 0.02% B. Braun 1 Bag 100ml

- Brand: B.BRAUN MILANO SpA
- Product Code: 975922966
- EAN: 4030539140232
- Availability: In 10 - 14 Days
- Purchase 3 items for 45.33€ each
- Purchase 4 items for 44.41€ each
- Purchase 5 items for 43.48€ each
Uro-tainer Poliesanide 0.02% B. Braun Sterile System
Patients with indwelling urinary catheters are at high risk of developing complications such as urinary tract infections and catheter fouling resulting in obstruction. These complications occur in 70% of patients with a catheter and can lead to blockage and loss of urine. Most patients with an indwelling catheter are elderly or have long-term disabilities, which can have a significant impact on their quality of life as catheter removal can be painful.
Uro-Tainer Poliesanide 0.02% is a sterile, ready-to-use and disposable system for the maintenance of indwelling catheters. It is used for the decolonization of bacteria from the transurethral or suprapubic catheter by mechanical removal.
Bacterial colonization of bladder catheters is favored by the formation of biofilms that protect microorganisms and hinder their elimination. Bacteria protected by biofilms are more resistant to antibiotic therapies. The usual therapeutic dosages of antibiotics have little or no effect on bacteria present in the form of biofilms.
To prevent the onset and regrowth of the biofilm, regular treatments with Uro-Tainer Poliesanide 0.02% can be effective thanks to its anti-adhesive effect which prevents microorganisms from adhering to the catheter surface to form colonies. Polyhexanide (PHMB) is already successfully used in wound care to promote bacterial decolonization and prevent biofilm formation.
PHMB is active against gram-, gram +, fungi and yeasts including MRSA (Methicillin Resistant Staphylococcus Aureus), VRE (Vancomycin Resistant Enterococcus), ESBL (Extended-Spectrum Beta- Lactamase), Escherichia Coli, Pseudomonas aeruginosa, Staphylococcus aureus, pneumoniae, etc. PHMB is not absorbed by cells and tissues, it does not interfere, therefore, with cellular metabolism, it is non-toxic, it is hypoallergenic, there is no evidence of any bacterial resistance and can also be used in the long term.
The tolerability of Uro-Tainer Poliesanide 0.02% allows you to perform, as needed, up to two treatments per day.
Technical features
Uro-Tainer Poliesanide 0.02% consists of a heat-sealed bag with a capacity of 100 ml, connected to a flexible flow tube equipped with a closing clamp, which ends with a universal connector equipped with a safety seal. The soft and transparent bag allows you to evaluate the presence of debris. The conical connector can be connected with the conical fittings of indwelling catheters. The system is enclosed in a protective plastic jacket. The use of Uro-Tainer Poliesanide 0.02% takes place in a closed circuit without the possibility of air inlet or outlet.
Composition
| Composition for 100 ml | polyhexamethylene biguanide (polyhexanide) 0.02 g, sorbitol in water for injections 5.0 g |
| Composition of the bag | bag in Nexcel M132A, a totally recyclable PVC-free polyolefin film |
| Clamp, cone and flow tube composition | polypropylene |
| Bag capacity | 100 ml |
| Primary packaging | heat-sealed polypropylene bag |
How to use
The recommended frequency of use should not exceed once or twice a day. Although adverse events are not expected with more frequent use, no clinical data regarding overdose are available.
Warnings
PHMB can cause allergic reactions such as itching and rashes. In rare cases (less than 1 case in 10,000) anaphylactic shock has occurred. The leakage of fluid from the urethra during irrigation indicates that the catheter may no longer be in the bladder, if the fluid does not flow the catheter could be blocked and therefore needs to be replaced.
Uro-Tainer Poliesanide 0.02% must not be used:
- in case of hypersensitivity to PHMB, chlorhexidine or the excipient of the solution
- in case of cystitis or other urogenital pathologies that cause haematuria
- in the days following bladder or urinary tract surgery
- avoid contact with open wounds, inner and middle ear, central nervous system, eyes, hyaline cartilage and meninges.
Do not use for intravenous infusion and injections. The solution is not a carrier for additives, do not use as a solvent of drugs. Use only if the liner, contents and sealing cap are intact. There is no evidence to indicate a mutagenic or embryotoxic effect of the product components. The absence of systemic absorption of polyhexanide makes transmission of the components to breast milk unlikely. Due to the lack of ad hoc trials and long-term clinical experience with pregnant, lactating, newborn and infants, this product should only be used after careful medical consultation.
storage
Store at room temperature, away from heat sources.
Validity for intact packaging: 36 months.
Format
Packaging 1 Bag of 100 ml.